After an introductory article on clinical evaluation and a second on the application of article 61(10) of the European regulation, it's time to present 5 Tips that I use every time I write a clinical evaluation plan and report.
These five tips will help you save time and find quality, relevant data.
The first piece of advice is to search for similar devices via databases such as EUDAMED.
If you're European, you're probably familiar with EUDAMED, the database that will (one day?) provide access to information on medical devices available on the market. Among the modules already present, there's one that allows you to " search for IUDs and data concerning the device ".
A little tip To find similar products, use the device nomenclature section and enter the EMDN code of your product. To filter more results, you can use the sections: device type, destination, or even risk class. You'll then come across a list of devices matching your search.
Be careful, though, because depending on the code you use, you may come across products that are very different from yours. A little Internet research on the device is often necessary to check that it really is similar to yours.
In the same vein as the first tip, even if your device is not registered in the USA, similar products may be present. Two databases are of interest: GUDID and certain modules of the FDA website.
GUDID looks like a classic database, with Booleans and keywords such as the type of product used, the name of a competitor or the GMDN or "Product code".
The modules for the FDA site of interest for searching for competitors or similar products are the following:
A little tip When I don't have the FDA product code, I use the GUDID to find them. I'll use the name of a competing manufacturer on the market, the GMDN code, the class etc...
Next, I use the FDA's product classification database and enter the product code I'm looking for. This will show me the type of submission for the associated code (510K or PMA).
And finally, I use the corresponding submission database, type in the associated product code, and I'll get the list of products registered for that code.
Please note, however, that not all registered products will necessarily be similar to yours - they're just part of the same "family".
To find potential vigilances concerning similar products, you have the option of going to the websites of competent authorities such as ANSM in France, and searching in the safety information for competing products.
You can also use the FDA's MAUDE module (Manufacturer and User Facility Device Experience). As with the previous module presented in tip #2, searches are performed using criteria such as product code. You can also enter product names, manufacturer names, search dates, etc.
You'll then be taken to a list where you can see reports and detailed descriptions of vigilance.
As mentioned in Article 2 of European Regulation 2017/745 on medical devices as well as MDCG Guide 2021-6 (Rev.1), a performance is the ability of a device to achieve its intended purpose.
Clinical performance, on the other hand, is the ability of a device to achieve its intended purpose, leading to a clinical benefit.
At Cisteo, we are particularly interested in categorizing the product's claimed performance, to distinguish explicitly between technical and clinical performance.
Although the term technical performance is not mentioned either in Regulation (EU) 2017/745 or in the MDCG guides, technical performance could be defined by the ability of a device to achieve its intended purpose and whose demonstration is based solely on the results of non-clinical test methods (such as preclinical evaluation, test benches, ...).
By categorizing performance in this way, you can more easily distinguish which clinical strategy should be applied to your medical device (based on Article 61(10) of Regulation (EU) 2017/745, equivalence or clinical data specific to the medical device under evaluation).
As part of a clinical investigation, this categorization could also help you determine which performances to evaluate.
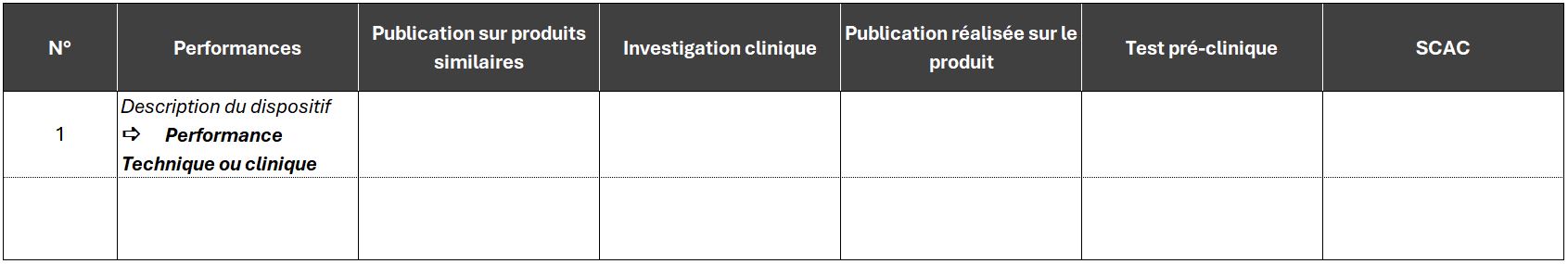
The clinical evaluation plan and report are documents containing a great deal of information, and can easily run to over 100 pages.
As with any document that forms part of technical documentation, make sure you break it down into sections, and don't hesitate to use tables to summarize your information. This will make the document easier to read.
Example of a summary presentation to demonstrate the performance of a medical device:
If you need help in drafting your clinical evaluation plan or report, or simply need to have it proofread, we're here to help.
GOOD TO KNOW: Our assistance is eligible for the Diag DM aid scheme, which finances 50% of the cost of the service.

